Femtosecond spectroscopy and TD-DFT calculations of CuCl42− excited states†
Abstract
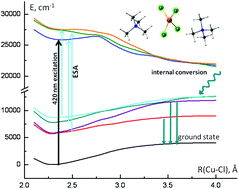
Photoinduced processes of tetrahexylammonium tetrachlorocuprate [(C6H13)4N]2CuIICl4 in chloro-organic solvents were investigated by steady state photolysis and femtosecond transient absorption spectroscopy. The quantum yield of photoreduction of CuCl42− was estimated to be about 1%; the process resulted in the formation of the copper(I) chlorocomplex CuICl32− and a chlorine atom. Femtosecond laser photolysis with a 422 nm, 40 fs pulse revealed a three-exponential decay of the LMCT excited state of [(C6H13)4N]2CuCl4. A global fitting SVD analysis of the femtosecond transient spectra suggested three relaxation times, ∼400 fs, ∼1.4 ps and ∼5.8 ps. Oscillations in transient absorption kinetic traces were documented for CuCl42− solutions in 2-chlorobutane. The oscillation Fourier transform analysis of the oscillations and linear predictive singular value decomposition revealed peaks at 283 cm−1 (damping time ∼600 fs) and 181 cm−1 (damping time ∼400 fs). These peaks can be tentatively attributed to νs(Cu–Cl) symmetric stretching frequency A1 and T2 reflecting excited state vibrational coherence. Quantum chemical calculations suggest a possible scheme for relaxation pathways in CuCl42−. The observed transient excited state absorption bands agree semiquantitatively with the calculated transition bands of CuCl42−.
- This article is part of the themed collection: Spectroscopy of Inorganic Excited States

 Please wait while we load your content...
Please wait while we load your content...