Antiferromagnetic Cu–Gd interactions through an oxime bridge†
Abstract
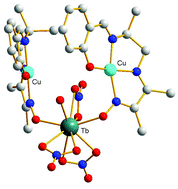
The copper complex of a polydentate non-symmetrical Schiff base ligand [LCu]2, prepared by template synthesis, has been reacted with the series of lanthanide ions. This complex used as a ligand possesses two functions (phenol and oxime) able to coordinate the Ln ions and, according to the Ln ion, three types of complexes are obtained. From La to Eu, trinuclear [(LCu)2Ln(NO3)3] complexes with a double phenoxo–oximato bridge were isolated. From Gd to Ho, the complexes [(LCu)2Ln(NO3)3(H2O)] are still trinuclear, with a supplementary water molecule linked to the Ln ion but the CuII and LnIII ions are only bridged by the oximato (N–O) pair, the phenoxo oxygen atom being hydrogen-bridged to the Ln-coordinated water molecule. Then, with heavier Ln ions, dinuclear [(LCu)Ln(NO3)3(H2O)2] complexes are characterized. The magnetic study demonstrates that the oximato bridge is responsible for the antiferromagnetic character of the Cu–Gd interaction, with JCuGd = −0.63 cm−1 in [(LCu)2Gd(NO3)3(H2O)], in contrast to the ferromagnetic Cu–Gd interaction induced by the single oxygen atom phenoxo bridge.

 Please wait while we load your content...
Please wait while we load your content...