Dehydrogenation of hydrocarbons with metal-carbon multiple bonds and trapping of a titanium(ii) intermediate†
Abstract
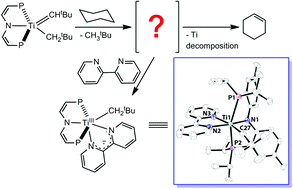
Reacting (PNP)Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHtBu(CH2tBu) with 2,2′-bipyridine (bipy) in cyclohexane or heptane results in dehydrogenation, cleanly producing cyclohexene and 1-heptene, respectively, and a TiII intermediate that is trapped by bipy to produce [(PNP)TiIII(CH2tBu)(bipy˙−)] (1). This titanium(II) intermediate reduces the bipy ligand upon coordination to form a TiIII center, where the unpaired electron is antiferromagnetically coupled to the electron of the reduced [bipy˙−] π-radical moiety, giving an overall diamagnetic species. Complex 1 has been characterized by NMR and UV-vis spectroscopies as well as single crystal X-ray diffraction studies.
CHtBu(CH2tBu) with 2,2′-bipyridine (bipy) in cyclohexane or heptane results in dehydrogenation, cleanly producing cyclohexene and 1-heptene, respectively, and a TiII intermediate that is trapped by bipy to produce [(PNP)TiIII(CH2tBu)(bipy˙−)] (1). This titanium(II) intermediate reduces the bipy ligand upon coordination to form a TiIII center, where the unpaired electron is antiferromagnetically coupled to the electron of the reduced [bipy˙−] π-radical moiety, giving an overall diamagnetic species. Complex 1 has been characterized by NMR and UV-vis spectroscopies as well as single crystal X-ray diffraction studies.
- This article is part of the themed collection: The Carbon–Metal Bond and C–H Metalation

 Please wait while we load your content...
Please wait while we load your content...