Aqueous stability of alumina and silica perhydrate hydrogels: experiments and computations†
Abstract
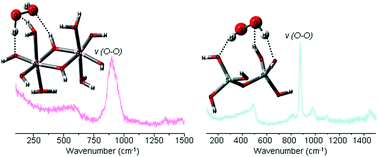
Alumina and silica perhydrate hydrogels were synthesized. Raman spectroscopy and solid 27Al MAS NMR confirmed alumina perhydrate formation. Thermal and aqueous stability of alumina and silica perhydrates was studied, and they showed exceptionally high stabilities. Alumina perhydrate retained some of the hydrogen peroxide even at 170 °C, higher than any other reported perhydrate, whereas the silica perhydrate lost its hydrogen peroxide content already at 90 °C. The silica perhydrate lost all its peroxide content upon immersion in water, whereas the alumina perhydrate was stable under near-neutral pH conditions. A computational study was conducted in order to glean molecular insight into the observed thermal and aqueous stability of alumina compared to silica perhydrate. Comparison of the hydrogen bond features and the stabilization energies of the hydrate and perhydrate of silica and alumina revealed a higher preference for hydrogen peroxide over water by alumina relative to silica. This is shown to be due to hydrogen peroxide being a better hydrogen donor than water and due to the superior hydrogen accepting propensity of alumina compared to silica.

 Please wait while we load your content...
Please wait while we load your content...