Comparative photo-release of nitric oxide from isomers of substituted terpyridinenitrosylruthenium(ii) complexes: experimental and computational investigations†
Abstract
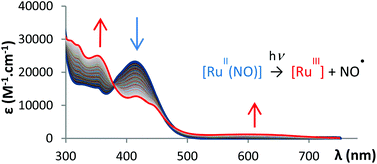
The 4′-(2-fluorenyl)-2,2′:6′,2′′-terpyridine (FT) ligand and its cis(Cl,Cl)- and trans(Cl,Cl)-[RuII(FT)Cl2(NO)](PF6) complexes have been synthesized. Both isomers were separated by HPLC and fully characterized by 1H and 13C NMR. The X-ray diffraction crystal structures were solved for FT (Pna21 space group, a = 34.960(4), b = 5.9306(7), c = 9.5911(10) Å), and trans(Cl,Cl)-[RuII(FT)Cl2(NO)](PF6)·MeOH (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, a = 10.3340(5), b = 13.0961(6), c = 13.2279(6) Å, α = 72.680(2), β = 70.488(2), γ = 67.090(2)°). Photo-release of NO˙ radicals occurs under irradiation at 405 nm, with a quantum yield of 0.31 and 0.10 for cis(Cl,Cl)-[RuII(FT)Cl2(NO)](PF6) and trans(Cl,Cl)-[RuII(FT)Cl2(NO)](PF6), respectively. This significant difference is likely due to the trans effect of Cl−, which favors the photo-release. UV-visible spectroscopy and cyclic voltammetry indicate the formation of ruthenium(III) species as photoproducts. A density functional theory (DFT) analysis provides a rationale for the understanding of the photo-physical properties, and allows relating the weakening of the Ru–NO bond, and finally the photo-dissociation, to HOMO → LUMO excitations.
space group, a = 10.3340(5), b = 13.0961(6), c = 13.2279(6) Å, α = 72.680(2), β = 70.488(2), γ = 67.090(2)°). Photo-release of NO˙ radicals occurs under irradiation at 405 nm, with a quantum yield of 0.31 and 0.10 for cis(Cl,Cl)-[RuII(FT)Cl2(NO)](PF6) and trans(Cl,Cl)-[RuII(FT)Cl2(NO)](PF6), respectively. This significant difference is likely due to the trans effect of Cl−, which favors the photo-release. UV-visible spectroscopy and cyclic voltammetry indicate the formation of ruthenium(III) species as photoproducts. A density functional theory (DFT) analysis provides a rationale for the understanding of the photo-physical properties, and allows relating the weakening of the Ru–NO bond, and finally the photo-dissociation, to HOMO → LUMO excitations.

 Please wait while we load your content...
Please wait while we load your content...