Heterometallic aluminates: alkali metals trapped by an aluminium aryloxide claw†
Abstract
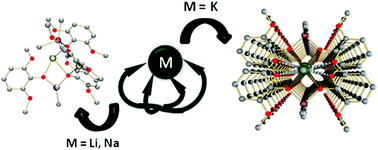
A series of heterometallic aluminium–alkali metal species [AlMMe2{2,6-(MeO)2C6H3O}2]n have been isolated for lithium, sodium and potassium. These compounds can be generated by the reaction of [AlMe2{2,6-(MeO)2C6H3O}]2 with the metallated phenol [M{2,6-(MeO)2C6H3O}]n or through the reaction of the mixture of AlMe3 and the appropriate alkali metal alkyl base with two equivalents of 2,6-dimethoxyphenol. In the heterometallic species obtained, the {AlMe2{2,6-(MeO)2C6H3O}2}− moiety is observed and could be described as a claw which fixes the alkali ion by the phenoxide oxygen atoms while the methoxy groups help to stabilize their coordination sphere. All compounds have been characterized by NMR spectroscopy and X-ray diffraction methods. Catalytic studies reveal that these compounds are active in ring-opening polymerization of L-lactide.
- This article is part of the themed collection: New Expeditions in Polar Organometallic Chemistry

 Please wait while we load your content...
Please wait while we load your content...