Kinetics of the autoxidation of sulfur(iv) co-catalyzed by peroxodisulfate and silver(i) ions†
Abstract
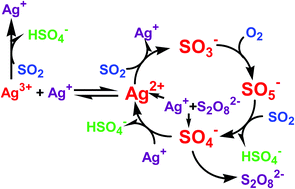
The kinetics and mechanism of the reaction between dissolved oxygen and sulfur(IV) was studied in aqueous acidic medium using co-catalysts peroxodisulfate and silver(I) ions. The presence of both catalysts was required to observe measurable rates in the studied process. The reaction rate was determined through following the UV-absorption of hydrated sulfur dioxide, and the trends were determined as a function of pH, reactant and catalyst concentrations. Individual kinetic curves under conditions where dissolved oxygen was the limiting reagent were close to zeroth-order. A chain mechanism with four chain carriers, sulfite, sulfate, peroxomonosulfate ion radical and silver(II) ion, is proposed to interpret all the kinetic and stoichiometric findings, and an explicit formula was obtained for the rate law. The role of the co-catalysts is to produce chain carriers, whereas silver(I) and silver(II) ions also participate in chain propagation steps. Further supporting evidence for the proposed mechanism was gained in laser flash photolysis studies, which showed that sulfate ion radical reacts quite rapidly with silver(I) ion.

 Please wait while we load your content...
Please wait while we load your content...