Formation of cyanates in low-valent uranium chemistry: a synergistic experimental/theoretical study†
Abstract
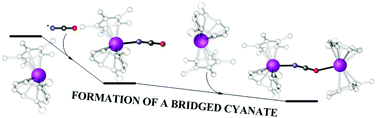
Computational studies on the reductive activation of a mixture of CO and NO by the U(III) complex [U(η-C8H6{SiiPr3-1,4}2)(η-Cp*)], which affords a mixture of [U(η-C8H6{SiiPr3-1,4}2)(η-Cp*)]2(μ-OCN)21 and [U(η-C8H6{SiiPr3-1,4}2)(η-Cp*)]2(μ-O) 2, show that the reaction proceeds via an initial attack of CO on a μ–η2:η2 coordinated NO, side-on bridged between two uranium centres. This leads to the formation of the bridging oxo complex 2 and the cyanate radical; coordination of the latter to the starting complex and dimerisation affords 1. The DFT studies also predict the existence of the monocyanate-bridged, mixed valence species [U(η-C8H6{SiiPr3-1,4}2)(η-Cp*)]2(μ-OCN) 3, which has now been experimentally observed.
- This article is part of the themed collection: Synergy between Experiment and Theory

 Please wait while we load your content...
Please wait while we load your content...