Synthesis, protonation equilibrium and peculiar thermal decomposition behavior of cyclo-tri-μ-imidotetraphosphate†
Abstract
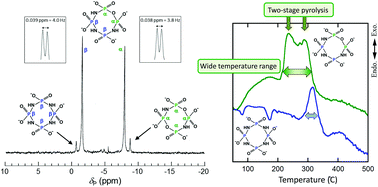
The synthesis and isolation of the sodium salt of cyclo-tri-μ-imidotetraphosphate, i.e. Na4cP4O9(NH)3·H2O, were achieved by the hydrolysis of Na4cP4O8(NH)4·2H2O under very weak acidic conditions, i.e. using 0.2 mol L−1 propionic acid and the pH-controlled recrystallization procedure. The purity of Na4cP4O9(NH)3·H2O was improved from 2% to 95% by the pH-controlled recrystallization only two times. The first protonation constants of a series of cyclo-μ-imidotetraphosphate anions, i.e. cP4O12−n(NH)n4− (n = 0, 2, 3, 4), were determined by potentiometric titration and 31P NMR chemical shift measurements in aqueous solution. Regardless of the paucity of the purity of trans-cP4O10(NH)24− anions, the protonation processes of all anions may be evaluated accurately without any previous purification, because the NMR signals corresponding to cP4O12−n(NH)n4− (n = 0, 2, 3, 4) anions are well resolved. The logarithmic first protonation constants increase with a “linear” increase in the number of imino groups which constitute the ligand molecules. Macroscopic protonation reactions could be divided into three microscopic protonation processes for –O–PO2–O–, –O–PO2–NH–, and –NH–PO2–NH– groups. The basicity of the –NH–PO2–NH– group is especially high, because the delocalization of H+ ions by lactam–lactim tautomerism on the whole ring molecule of cP3O6(NH)3 and cP4O8(NH)4 enhances the protonation of these ligands. In addition, also the concurrent change observed in the 31P NMR chemical shift values of the phosphorus nuclei in the –O–PO2–NH– and –NH–PO2–NH– groups of cP4O9(NH)34− anions suggested the effect of the lactam–lactim tautomerism. The intrinsic 31P NMR chemical shifts for the central phosphorus nuclei for –O–PO2–O–, –O–PO2–NH–, and –NH–PO2–NH– groups show a good proportional relationship with the number of nitrogen atoms bonded to the central phosphorus atoms. Two types of imino groups with mutually dissimilar chemical environments which are present in the Na4cP4O9(NH)3 molecule, that is –O–PO2–NH–PO2–NH– and –NH–PO2–NH–PO2–NH–, brought about a two-stage pyrolytic elimination of imino groups from the initial stage of combustion over a wide temperature range.

 Please wait while we load your content...
Please wait while we load your content...