Deprotonation/protonation-driven change of the σ-donor ability of a sulfur atom in iron(ii) complexes with a thioamide SNS pincer type ligand†
Abstract
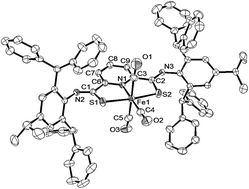
A new iron complex with a thioamide SNS pincer type ligand, [FeBr2(κ3-H2LDPM)] (κ3-H2LDPM = 2,6-bis(N-2,6-bis(diphenylmethyl)-4-isopropylphenylthioamide)pyridine), was synthesized. This complex reacts with NaH in THF to yield a unique Fe(II) complex with two THF molecules, [Fe(THF)2(κ3-LDPM)] (κ3-LDPM = 2,6-bis(N-2,6-bis(diphenylmethyl)-4-isopropylphenyliminothiolate)pyridine). The THF molecules of [Fe(THF)2(κ3-LDPM)] can be substituted with CO and CN-xylyl to give [Fe(CO)3(κ3-LDPM)] and [Fe(CN-xylyl)3(κ3-LDPM)], respectively. The complex [Fe(CN-xylyl)3(κ3-LDPM)] reacts with HBF4 to produce [Fe(CN-xylyl)3(κ3-H2LDPM)]2+ with protonated thioamide units. The differences of the IR spectra before and after protonation indicate that the major binding mode of CN-xylyl to iron(II) changes from π-back donation from metal to isocyanide to σ-donation from isocyanide to iron(II). This indicates that the σ-donor ability of the thioamide sulfur atom is tuned by deprotonation/protonation of thioamide.

 Please wait while we load your content...
Please wait while we load your content...