A DFT study of ruthenium pincer carboxylate complexes as potential catalysts for the direct carboxylation of arenes with CO2 – meridional versus facial coordination†
Abstract
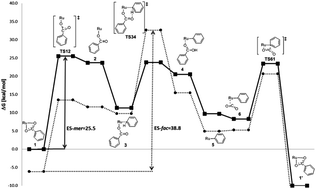
A recent DFT study of the ruthenium pincer benzoate complex [Ru(PNP)(PhCOO)2] I (PNP = 2,6-bis(diphenylphosphanyl)lutidine) in its meridional form has revealed mer-I to be a promising catalyst lead structure for the direct insertion of CO2 into the C–H bonds of arenes, such as benzene. After the successful synthesis of I, its solid state structure interestingly and unexpectedly showed the pincer ligand to adopt the facial rather than the meridional coordination mode. Recalculation of the catalytic cycle with fac-I including all relevant local minima and transition states revealed (a) fac-I to be significantly more stable (6.1 kcal mol−1) than mer-I, (b) that the energetic span (ES; i.e. the effective activation barrier) for the cycle with fac-I amounts to 38.8 kcal mol−1, while the cycle with mer-I has an ES of 25.5 kcal mol−1 only. These results are a hint that fac-I is catalytically inactive. Experimental testing of fac-I showed indeed no product formation, which is in full accordance with the computations. To reduce the spatial flexibility of the pincer ligand, its CH2 groups were replaced by O atoms. The resulting complex [Ru(PONOP)(PhCOO)2] II (PONOP = 2,6-bis(diphenylphosphinito)pyridine) was used for the calculation of the catalytic cycle in benzene as the solvent. Gratifyingly, the starting complex mer-II is more stable than fac-II by 1.9 kcal mol−1 in benzene as the solvent. Consequently, mer-II should be available experimentally. As with fac-I, also fac-II generates a catalytic cycle with a high ES (37.1 kcal mol−1), while mer-II generates a cycle with a significantly lower ES (27.2 kcal mol−1) indicating mer-II to be a potentially active catalyst. A possible explanation of the much lower ES in the case of the meridionally coordinated species is found in the stronger interaction of the substrate with the metal center in the arene-σ-bond complex. As a result the issue that is created by the mer/fac isomerism can be resolved by creating spatially less flexible structures.
- This article is part of the themed collection: Synergy between Experiment and Theory

 Please wait while we load your content...
Please wait while we load your content...