An exemplary relationship between the extent of cofacial aggregation and fluorescence quantum yield as exhibited by quaternized amphiphilic phthalocyanines†
Abstract
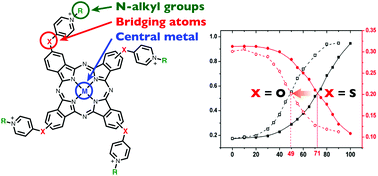
The aggregation equilibrium of a series of positively-charged water-soluble phthalocyanines (Pcs) was studied in aqueous solution by means of absorption and fluorescence techniques. The aggregation equilibrium in water depends on the bridging atoms between the pyridyl groups and the Pc core, and on the central metal of the Pcs, while N-alkyl groups are virtually uninvolved in the aggregation properties. Thus, in the water–methanol mixture, the aggregation tendency increased in the order of Zn < Cu ≤ Ni, Pd when the ligand is the same, while the bridging element sulfur gives much more propensity for aggregation compared to oxygen. The fluorescence quantum yields of the Pcs showed an excellent correlation with the extent of aggregation.

 Please wait while we load your content...
Please wait while we load your content...