Cycloalkyl-based unsymmetrical unsaturated (U2)-NHC ligands: flexibility and dissymmetry in ruthenium-catalysed olefin metathesis†
Abstract
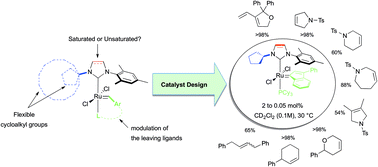
Air-stable Ru-indenylidene and Hoveyda-type complexes bearing new unsymmetrical unsaturated N-heterocyclic carbene (U2-NHC) ligands combining a mesityl unit and a flexible cycloalkyl moiety as N-substituents were synthesised. Structural features, chemical stabilities and catalytic profiles in olefin metathesis of this new library of cycloalkyl-based U2-NHC Ru complexes were studied and compared with their unsymmetrical saturated NHC-Ru homologues as well as a set of commercially available Ru-catalysts bearing either symmetrical SIMes or IMes NHC ligands.

 Please wait while we load your content...
Please wait while we load your content...