Unexpected bond activations promoted by palladium nanoparticles†
Abstract
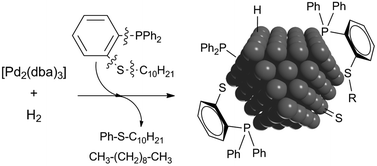
Thioether-phosphines, 1 and 2, were applied for the stabilisation of palladium nanoparticles (PdNPs) synthesised by a bottom-up methodology, using [Pd2(dba)3] as an organometallic precursor. For the phenyl containing ligand 1, small (dmean = 1.6 nm), well-defined and dispersed nanoparticles were obtained; however, ligand 2 involving a long alkyl chain led to agglomerates. NMR and GC-MS analyses throughout the synthesis of the nanomaterials revealed partial cleavage of ligands by C–S and C–P bond activations, and XPS spectra of the isolated nanoparticles indicated the presence of both thioether-phosphines and their fragments on the metallic surface. Reactivity studies of molecular palladium systems as well as on extended palladium surfaces pointed out that cluster entities are responsible for C-heteroatom activations, triggering structure modifications of stabilisers during the synthesis of PdNPs.

 Please wait while we load your content...
Please wait while we load your content...