Fractionation of rare-earth metallofullerenes via reversible uptake and release from reactive silica†
Abstract
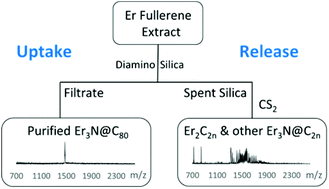
Minimal research exists for non-chromatographic separations of rare-earth metallofullerenes containing di-metallic (M2), di-metallic carbide (M2C2), and tri-metallic nitride (M3N) clusters trapped inside fullerene cages. Herein, we demonstrate a non-HPLC method (i.e., SAFA, Stir and Filter Approach) for purifying Er3N@Ih-C80, a rare-earth, metallic nitride clusterfullerene. We describe a strategic method that chemically releases rare-earth metallofullerenes (e.g., M2@C2n, M3N@C2n) trapped by aminosilica during SAFA. Recovery of metallofullerenes from spent silica represents a “green approach” because the spent silica and its useful, immobilized rare-earth metallofullerenes would have been discarded as waste material. We observe selectivity during metallofullerene uptake to aminosilica and also during its release from spent silica via addition of CS2. We describe a procedure to obtain samples enriched in M2 and M3N endohedrals. M2C2n fractions from our SAFA release process contain a wide range of higher metallofullerenes (e.g., Gd2C90–Gd2C140 or Er2C76–Er2C122). It is facile to obtain samples enriched in M3N@C82–M3N@C92. Note that unreacted M3N@C80 remains in the filtrate. The strategy for handling rare-earth metallofullerenes with different degrees of reactivity toward aminosilica is also discussed.
- This article is part of the themed collection: Organometallic and coordination chemistry of carbon nanomaterials

 Please wait while we load your content...
Please wait while we load your content...