Synthesis and characterisation of magnesium complexes containing sterically demanding N,N′-bis(aryl)amidinate ligands†
Abstract
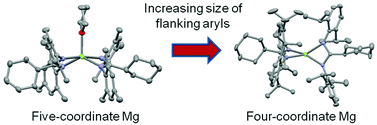
Condensation reactions of carboxylic acids and anilines in the presence of polyphosphoric acid trimethylsilyl ester (PPSE) afforded a range of sterically demanding N,N′-bis(aryl)amidines, RN{C(R′)}N(H)R [R = Mes (Mes = 2,4,6-trimethylphenyl), R′ = Cy (Cy = cyclohexyl) L1H; R = Dipp (Dipp = 2,6-diisopropylphenyl), R′ = Cy L2H; R = Mes, R′ = Ph L3H; R = Dipp, R′ = Ph L4H; R = Mes, R′ = Dmp (Dmp = 3,5-dimethylphenyl) L5H; R = Dipp, R′ = Dmp L6H; R = Dmp, R′ = Cy L7H]. Amidines L1H–L7H have been characterised spectroscopically, and for L5H and L6H, by X-ray crystallography. Treatment of the amidines with di-n-butylmagnesium in THF solution afforded the monomeric magnesium bis(amidinates) [Mg(L1)2(THF)] 1, [Mg(L2)2] 2, [Mg(L3)2(THF)] 3, [Mg(L5)2(THF)] 5, [Mg(L6)2] 6, [Mg(L7)2] 7, and the magnesium mono(amidinate) complex [Mg(L4)(nBu)] 4. These complexes have been characterised spectroscopically, with 1–3, 5 and 6 also being structurally authenticated. Comparison of the magnesium bis(amidinate) complexes reveals that the steric bulk of the amidinate ligand influences both the solid state structure and solution behaviour of these complexes.

 Please wait while we load your content...
Please wait while we load your content...