Effect of robust π–π stacking synthon on the formation of mercury coordination compounds; an unusual pseudo-square planar geometry†
Abstract
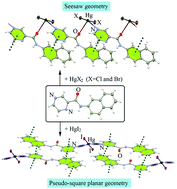
In this study, three Hg(II) complexes, [HgCl2(L2-naph)]n, 1, [HgBr2(L2-naph)]n, 2 and [HgI2(L2-naph)2], 3 where L2-naph is N-(naphthalene-2-yl)pyrazine-2-carboxamide ligand have been synthesized and characterized. X-ray single crystal diffraction analysis of these compounds reveals that 1 and 2 are isostructural coordination polymers and 3 is a discrete compound. In comparison to homologue complexes containing N-(naphthalene-1-yl)pyrazine-2-carboxamide ligand, interestingly, structural analysis clearly shows that displacing substituent position plays an important role in the formation of the supramolecular organization of molecular complexes. The common feature in the crystal packing of these complexes is that there is a strong tendency to form π–π stacking interaction between pyrazine and naphthalene rings. These π–π stacking interaction synthons affect the coordination geometry and structural assembly. Also, theoretical methods show the π–π stacking interaction energies within a range of −64.13 to −70.51 kJ mol−1. It is notable that in 3, cooperation of intermolecular π–π stacking synthon and intramolecular C–Hpyz⋯I–Hg hydrogen bond resulted in the formation of unusual pseudo-square planar geometry around the Hg(II) center. This study reveals an undeniable contribution of π–π stacking interaction to the organization and stabilization of some of the crystal structures reported here.

 Please wait while we load your content...
Please wait while we load your content...