Synthesis and characterization of copper(ii) 4′-phenyl-terpyridine compounds and catalytic application for aerobic oxidation of benzylic alcohols†
Abstract
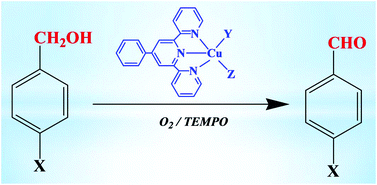
The reactions between 4′-phenyl-terpyridine (L) and nitrate, acetate or chloride Cu(II) salts led to the formation of [Cu(NO3)2L] (1), [Cu(OCOCH3)2L]·CH2Cl2 (2·CH2Cl2) and [CuCl2L]·[Cu(Cl)(μ-Cl)L]2 (3), respectively. Upon dissolving 1 in mixtures of DMSO–MeOH or EtOH–DMF the compounds [Cu(H2O){OS(CH3)2}L](NO3)2 (4) and [Cu(HO)(CH3CH2OH)L](NO3) (5) were obtained, in this order. Reaction of 3 with AgSO3CF3 led to [CuCl(OSO2CF3)L] (6). The compounds were characterized by ESI-MS, IR, elemental analysis, electrochemical techniques and, for 2–6, also by single crystal X-ray diffraction. They undergo, by cyclic voltammetry, two single-electron irreversible reductions assigned to Cu(II) → Cu(I) and Cu(I) → Cu(0) and, for those of the same structural type, the reduction potential appears to correlate with the summation of the values of the Lever electrochemical EL ligand parameter, which is reported for the first time for copper complexes. Complexes 1–6 in combination with TEMPO (2,2,6,6-tetramethylpiperidinyl-1-oxyl radical) can exhibit a high catalytic activity, under mild conditions and in alkaline aqueous solution, for the aerobic oxidation of benzylic alcohols. Molar yields up to 94% (based on the alcohol) with TON values up to 320 were achieved after 22 h.

 Please wait while we load your content...
Please wait while we load your content...