A speciation study of sulfur(iv) in aqueous solution
Abstract
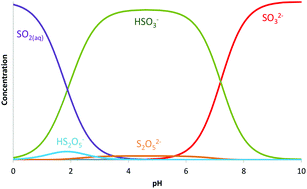
In the current work ultraviolet spectrophotometric titrations at different S(IV) concentrations have been globally analysed using the entire spectral dataset to determine the complete speciation of S(IV) in aqueous solution over a large pH range (from 9.6 to 1). As a result, the dimerisation constant for the formation of disulfite from hydrogen sulfite has been accurately determined. Further, protonated disulfite has been identified and quantified for the first time. In addition, the molar absorptivities of all S(IV) species are also reported over the studied wavelength range.

 Please wait while we load your content...
Please wait while we load your content...