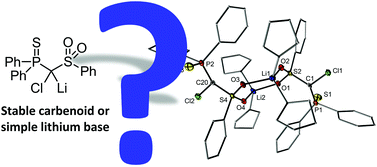
On the structure and ambiphilicity of a sulfonyl substituted α-chloro lithium base†
Abstract
An α-chloro lithium base stabilised by a sulfonyl and thiophosphinoyl moiety was selectively prepared by lithiation of its protonated precursor and oxidation of the corresponding dilithio methandiide. The carbenoid-like compound was found to be remarkably stable even at room temperature and thus allowed for its spectroscopic characterisation in solution and in the solid state. Its ambiphilic nature was tested and compared with typical carbenoids both experimentally and by computational methods. The electronic stabilisation results in its thermal stability but considerably reduces the ambiphilic character limiting the reactivity patterns generally observed for lithium carbenoids.
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...