Syntheses and structural characterization of o-carboranylamides with direct cage–amide bond†
Abstract
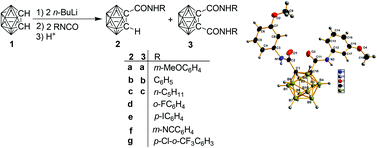
Reactions of lithio-o-carborane with isocyanates under various conditions were studied, and the structural features of the resulting carboranylamides are described. The reactions of o-carborane (o-C2B10H12), n-BuLi (two equiv.) and two equiv. of (substituted) phenylisocyanate, pentylisocyanate and p-ethylphenylthioisocyanate in diethyl ether, respectively, led, after workup, to the corresponding mono-substituted carboranylamide 2a–g and carboranylthioamide 5 in low to moderate yields, and only with RNCO (R = Ph, m-MeOC6H4, pentyl) could disubstituted products 3a–c be isolated. The reaction with phenylisocyanate afforded the mono-amide and di-amide products in a ratio of approximately 1 : 2, whereas in the other two reactions the ratios are approximately 4 : 1 and 3 : 2, respectively. In tetrahydrofuran all the reactions attempted with RNCO (R = Ph, p-IC6H4, m-NCC6H4 and pentyl) gave more monoamide products than those in diethyl ether. With phenylisocyanate no diamide product was isolated and with pentylisocyanate the ratio between monoamide and diamide is approximately 3.5 : 1. The new carboranylamides were characterized by means of elemental analyses, IR and NMR spectroscopy and mass spectrometry, as well as single-crystal X-ray diffraction analyses of 2a–f, 3a and 5.
- This article is part of the themed collection: Carboranes

 Please wait while we load your content...
Please wait while we load your content...