Fluorine insertion into the Ruddlesden–Popper phase La2BaFe2O7: the structure and magnetic properties of La2BaFe2O5F4
Abstract
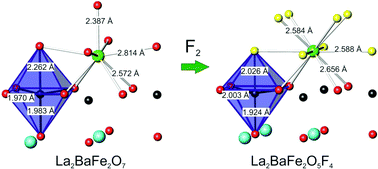
Fluorination of the n = 2 Ruddlesden–Popper phase La2BaFe2O7 occurs at ∼300 °C in flowing 10% F2 in N2 to form La2BaFe2O5F4. This oxide fluoride contains 2F− ions in interstitial sites within the rocksalt regions and 2F− ions that have substituted for O2− ions in apical sites within the rocksalt layers. The fluorination results in an expansion along c of 7.6% to yield a tetragonal unit cell of dimensions a = 3.96237(7) Å, c = 22.3972(5) Å. The structure and magnetic properties have been examined by Mössbauer spectroscopy, neutron powder diffraction and magnetic susceptibility measurements. La2BaFe2O5F4 becomes antiferromagnetically ordered at temperatures below ∼500 K, and the magnetic order shows a striking resemblance to that observed in La2BaFe2O7. The magnetic moments on Fe3+ are perpendicular to [001] and aligned along ±{100} directions above 300 K, but at temperatures below 200 K, they rotate by 45° to lie along ±{110}. Mössbauer spectroscopy suggests the presence of Fe3+ within the primary phase, but also indicates that fluorination results in some particle fragmentation to form a paramagnetic component of the fluorinated material.

 Please wait while we load your content...
Please wait while we load your content...