A novel intermediate in the LiAlH4–LiNH2 hydrogen storage system†
Abstract
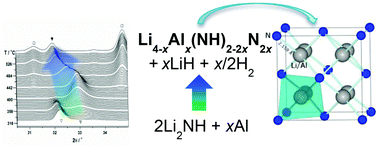
The decomposition pathways for the composite LiAlH4–LiNH2 in different ratios of (1 : 1), (1 : 1.5), (1 : 2) and (1 : 2.5) have been systematically studied using in situ synchrotron radiation powder X-ray diffraction (SR-PXD) as well as simultaneous thermogravimetric analysis and differential scanning calorimetry coupled with mass spectroscopy. The study reveals that LiAlH4 decomposes in two steps to LiH, Al and H2 and, subsequently, the produced LiH reacts with LiNH2 forming Li2NH and H2. A new intermediate, Li4−xAlx(NH)2–2xN2x, is observed during the decomposition of LiAlH4–LiNH2 (1 : 1.5), (1 : 2) and (1 : 2.5), formed from Li2NH and Al prior to the formation of Li3AlN2. Li4−xAlx(NH)2–2xN2x is characterized by Rietveld refinement of SR-PXD data and solid-state 27Al MAS NMR spectroscopy (chemical shift, δ(Al) = 125 ppm) and both techniques reveal a maximum value for x of ∼0.10, i.e., Li3.90Al0.10(NH)1.80N0.20. The solid solution Li4−xAlx(NH)2–2xN2x crystallizes in a cubic unit cell, a = 4.9854(7) Å with space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, similar to the crystal structure for Li2NH and is a rare type with both cation and anion disorder. For LiAlH4–LiNH2 (1 : 1) 8.7 wt% of H2 is released during heating from RT to 500 °C, while for LiAlH4–LiNH2 composites with molar ratios of LiNH2 higher than 0.5 the release of both H2 and NH3 is observed.
m, similar to the crystal structure for Li2NH and is a rare type with both cation and anion disorder. For LiAlH4–LiNH2 (1 : 1) 8.7 wt% of H2 is released during heating from RT to 500 °C, while for LiAlH4–LiNH2 composites with molar ratios of LiNH2 higher than 0.5 the release of both H2 and NH3 is observed.

 Please wait while we load your content...
Please wait while we load your content...