Anion effect on the control of morphology for NiC2O4·2H2O nanostructures as precursors for synthesis of Ni(OH)2 and NiO nanostructures and their application for removing heavy metal ions of cadmium(ii) and lead(ii)†
Abstract
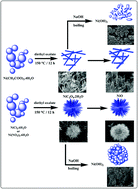
In this work, one-dimensional (1-D) and three-dimensional (3-D) nickel oxalate dihydrate nanostructures have been selectively prepared by a one-step and surfactant-free solvothermal approach. This preparation procedure can control the product morphology by varying the reaction temperature and anions such as nitrates, acetates, chlorides and sulfates. The morphology of nickel oxalate dihydrate changed from non-uniform sheets and ribbons to nanorods with higher temperatures while changing the initial anion converted nanorods to dandelion-like nanostructures. Field emission scanning electron microscopy (FESEM) shows that the sizes of the nickel oxalate dihydrate nanorods are about 3 μm in length and the dandelion-like nanostructures have an average diameter of around 10 μm. Furthermore, the adsorption of the heavy metal ions of cadmium(II) and lead(II) with nanostructured nickel compounds was investigated. The results indicate the NiO and Ni(OH)2 nanostructures can effectively adsorb cadmium(II) and lead(II) ions from aqueous solution.

 Please wait while we load your content...
Please wait while we load your content...