An ab initio thermodynamics study of cobalt surface phases under ethanol steam reforming conditions†
Abstract
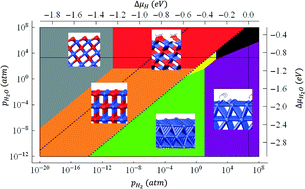
Co-based materials have emerged as promising ethanol steam reforming (ESR) catalysts in recent years. Both Co0 (Co metal) and Co2+ (CoO oxide) states were found to exist in the catalyst under reaction conditions and contribute to the catalytic activity, although their separate roles are still not fully understood. Density function theory (DFT) calculations were carried out to explore possible surface configurations based on the (100) and (111) facets of CoO. Ab initio atomistic thermodynamics was then applied to study the relative stability of various surface structures of Co0/Co2+ under ESR reaction conditions where H2O and H2 are the most abundant components in the gas phase. Based on the surface phase diagrams of CoO(100), CoO(111), and the general Co0/Co2+ catalyst, we found that the clean CoO(100) and OH*-covered CoO(111) are the most stable Co2+ surface configurations under ESR reaction conditions. We also suggest that a reducible support may be important in stabilizing Co2+ surfaces against reduction into metallic Co surfaces. The stable surface configurations of CoO identified in this paper can guide future DFT studies on the ESR catalytic activity of Co2+.
- This article is part of the themed collection: Catalysis in the USA

 Please wait while we load your content...
Please wait while we load your content...