Discrimination of the mechanism of CH4 formation in Fischer–Tropsch synthesis on Co catalysts: a combined approach of DFT, kinetic isotope effects and kinetic analysis†
Abstract
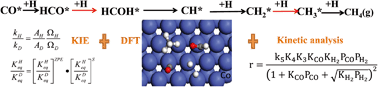
The mechanism of CH4 formation during Fischer–Tropsch synthesis on cobalt has been studied. DFT, kinetic isotope effect and kinetic analyses are combined to discriminate between the possible reaction routes of CH4 formation on Co catalysts. Nine direct reaction mechanisms were proposed from 21 elementary steps. They were first screened by DFT calculations in which the activation energies as well as the free energy profiles in each direct mechanism were compared, resulting in a reduction to six reaction mechanisms. Additional reduction was based on kinetic analysis where the reaction order was used as a descriptor. Subsequently, the kinetic isotope effect (KIE) values were calculated and compared to our previous SSITKA results. Finally, the dominating reaction route was suggested, which follows the initial elementary steps with H-assisted CO activation to form HCOH via HCO as an intermediate. It then proceeds through HCOH dissociation to CH followed by stepwise hydrogenation to CH4.
- This article is part of the themed collection: Mechanistic Studies in Catalysis

 Please wait while we load your content...
Please wait while we load your content...