Surface stabilities and NO oxidation kinetics on hexagonal-phase LaCoO3 facets: a first-principles study†
Abstract
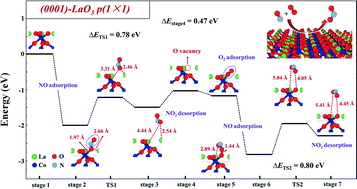
LaCoO3 perovskite has recently received great attention as a potential alternative to precious metal based NO oxidation catalysts. We report here a comprehensive first-principles study of the NO oxidation kinetics on differently re-constructed hexagonal-phase LaCoO3 facets. Among the 42 low-index facets considered, the (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 02) LaO-, (
02) LaO-, (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 104) O2- and (0001) LaO3-terminated facets were found to be thermodynamically stable and likely to be exposed in LaCoO3 oxide nanoparticles. Among these stable facets, the (0001) LaO3-terminated surface is catalytically most active towards NO oxidation, with the reaction proceeding through the mono-vacancy Mars–van Krevelen mechanism. Our study shed light on the atomistic scale NO oxidation mechanism on LaCoO3 facets and can aid further optimization of the catalyst.
104) O2- and (0001) LaO3-terminated facets were found to be thermodynamically stable and likely to be exposed in LaCoO3 oxide nanoparticles. Among these stable facets, the (0001) LaO3-terminated surface is catalytically most active towards NO oxidation, with the reaction proceeding through the mono-vacancy Mars–van Krevelen mechanism. Our study shed light on the atomistic scale NO oxidation mechanism on LaCoO3 facets and can aid further optimization of the catalyst.

 Please wait while we load your content...
Please wait while we load your content...