Cl− making overall water splitting possible on TiO2-based photocatalysts†
Abstract
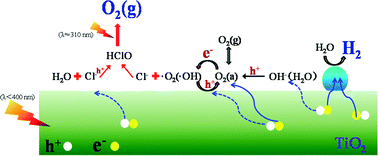
Overall water splitting on a TiO2-based photocatalyst has been extensively investigated. However, in most cases, the products are not in a stoichiometric ratio, thus the reaction is not really overall water splitting. In this work, we found that in the presence of Cl−, the evolution of O2 and H2 over Pt/TiO2 can be successfully achieved, and the activity can be enhanced up to 3 times compared to having no Cl− present. Furthermore, the H2 : O2 ratio can be close to 2.0, i.e. the stoichiometric ratio of overall water splitting. It is proposed that the Cl− ion is involved with the reaction intermediate of O2 evolution from water oxidation. Our work not only reported overall water splitting on a TiO2-based photocatalyst, but also provided experimental evidence for understanding the possible reaction process and the mechanism of photocatalytic water splitting.

 Please wait while we load your content...
Please wait while we load your content...