Toward a mechanistic understanding of oxidative homocoupling: the Glaser–Hay reaction†
Abstract
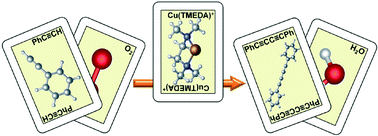
The copper-catalyzed oxidative homocoupling of terminal alkynes has been studied with DFT methods. The role of Cu(I) or Cu(II) as the initial oxidation state as well as the effect of the changes in the substrate and the base have been examined. Oxidants responsible for outer- and inner-sphere electron transfer processes have also been investigated. The Cu/O2 interactions, which arise when dioxygen is employed as the oxidant, have been studied explicitly to fully describe the 4-electron reduction process, providing a plausible mechanism that could serve as a model for other aerobic oxidative couplings. The obtained results completely agree with the reported experimental data: the computed free energy barriers are low enough for the reactions to proceed at room temperature, and electron-poor alkynes and stronger bases lead to faster reactions.

 Please wait while we load your content...
Please wait while we load your content...