Synthesis, acid properties and catalysis by niobium oxide nanostructured materials†
Abstract
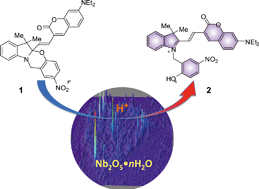
Several forms of niobium oxide were prepared, including nanostructured mesoporous materials, and their acidity properties were comprehensively investigated and compared with commercially available materials. The composites were characterized by a variety of techniques, including XRD, TEM, N2 adsorption and Hammett acid indicator studies. The acidity of the niobium oxide derivatives was also investigated by the ability of the materials to successfully promote the halochromic ring-opening of an oxazine-coumarin probe that was specifically designed for use in fluorescence imaging studies. The ring-opening reaction was easily monitored using UV-visible, fluorescence and NMR spectroscopy. Single molecule microscopy was employed to gain a more in-depth understanding of the niobium oxide acid catalysis pathway. Using this technique, the rate of niobium oxide mediated protonation was estimated to be 1.8 × 10−13 mol m−2 s−1. Single molecule analysis was also used to obtain a detailed map of Brønsted acid sites on the niobium oxide surface. The active sites, located by multiple blinking events, do not seem to be localized on any area of the material, but rather randomly distributed throughout the solid state surface. As the reaction proceeds, the sites with the highest acidity and accessibility are gradually consumed, making the next tier of acid sites available for reaction. The phenomenon was more closely characterized by using time lapsed reactivity maps.

 Please wait while we load your content...
Please wait while we load your content...