A reusable CuII based metal–organic framework as a catalyst for the oxidation of olefins
Abstract
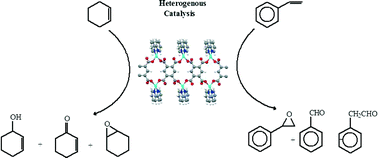
The metal–organic framework [Cu2(bipy)2(btec)]∞ was used as a heterogeneous catalyst in the liquid phase oxidation of styrene and cyclohexene with tert-butylhydroperoxide (TBHP) as the oxidant either in water–dichloroethane or n-decane medium. Four catalytic systems were tested and compared after 6 hours of reaction. The oxidation of styrene using [Cu2(bipy)2(btec)]∞ showed a competitive conversion, as compared with some CuII heterogeneous catalysts and MOF catalysts. Conversion between 61–45% in dichloroethane : TBHP : water at 75 °C was obtained, using molar ratios of substrate : catalyst from 200 to 2400. When the solvent was replaced by n-decane, the conversion remained similar, that is, between 57–46%, at the same mole ratio range of substrate : catalyst. Styrene oxidation in dichloroethane : TBHP : water [Cu2(bipy)2(btec)]∞ is not selective; the products are benzaldehyde, styrene epoxide and 1-phenylacetaldehyde in 37%, 35% and 29% yields, respectively. The oxidation of cyclohexene showed a conversion of 34–16% under the same experimental conditions in dichloroethane : TBHP : water at 75 °C, with the catalytic reaction presenting a higher selectivity for the formation of 2-cyclohexen-1-one as the product. When n-decane was used, the total conversion in cyclohexene oxidation decreased approximately by 10%, as compared to the dichloroethane : TBHP : water system. The activities reported for the Cu-MOF showed a very high selectivity for the allylic oxidation product. Leaching during the catalytic reaction was found to be negligible, and the structure was maintained during the four catalytic runs, as confirmed by powder X-ray diffraction analysis of the reused catalyst.

 Please wait while we load your content...
Please wait while we load your content...