Catalytic olefin epoxidation over cobalt(ii)-containing mesoporous silica by molecular oxygen in dimethylformamide medium†
Abstract
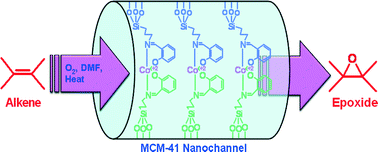
A cobalt(II) Schiff base complex has been immobilized onto the surface of Si–MCM-41 to prepare a new catalyst. The amine group-containing organic moiety 3-aminopropyl-triethoxysilane had first been anchored on the surface of Si–MCM-41 via a silicon alkoxide route. Upon condensation with salicylaldehyde, the amine group affords a bidentate Schiff-base moiety in the mesoporous matrix, which is subsequently used for anchoring of cobalt(II) centers. The prepared catalyst has been characterized by UV-vis, infrared (IR), EPR spectroscopic and small angle X-ray diffraction (XRD) analyses, and N2 sorption studies. The catalytic activity was tested in epoxidation reactions of olefinic compounds, including styrene and allyl alcohol, with molecular oxygen at atmospheric pressure in dimethylformamide medium in the absence of additional sacrificial reductant. The reactions seemed to proceed through a radical formation mechanism. The immobilized catalyst showed good activity and epoxide selectivity in the alkene epoxidation. Notably, the catalyst can be recovered and reused without any loss of activity.

 Please wait while we load your content...
Please wait while we load your content...