Deactivation studies of a carbon supported AuPt nanoparticulate catalyst in the liquid-phase aerobic oxidation of 1,2-propanediol†
Abstract
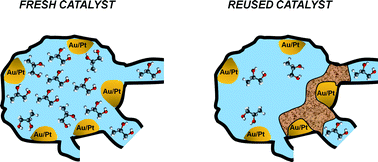
The aerobic oxidation of 1,2-propanediol in alkaline aqueous solvent over bimetallic AuPt/C catalysts has been studied and catalyst reusability has been assessed. A systematic decrease of catalytic conversion was observed after each reuse of the catalyst. In order to understand the causes of deactivation, the catalyst samples were characterised by N2 adsorption, temperature-programmed oxidation (TPO) and pulsed-field gradient nuclear magnetic resonance (PFG-NMR) diffusion measurements. The results revealed that the catalyst surface area and pore volume decrease significantly after each reuse of the catalyst. The intra-particle diffusion is characterised by two distinct diffusion regimes, a fast regime with self-diffusivities of 10−9–10−11 m2 s−1 and a slow diffusion regime, with values of self-diffusivities on the order of 10−11–10−13 m2 s−1. Self-diffusivity in the fast regime is assigned to diffusion within the mesoporous space of the catalyst. Self-diffusivity in the slow diffusion region is assigned to diffusion within the microporous space and decreases after each reuse of the catalyst in a trend similar to that of pore volume, suggesting that changes in catalyst porosity and pore structure affect molecular mobility within the micropores. TPO studies of these systems showed a different distribution of oxidation products in the reused catalyst samples compared to the fresh catalyst, which suggests changes of the combustion mechanism. Altogether, the results reveal that catalyst deactivation is caused by deposition and build-up of heavy molecular species on the catalyst surface, which reduce the catalyst porosity by pore blockage and narrowing of channels, which in turn affects the diffusion rate within the micropores.

 Please wait while we load your content...
Please wait while we load your content...