Metal-catalyzed activation of ethers via C–O bond cleavage: a new strategy for molecular diversity
Abstract
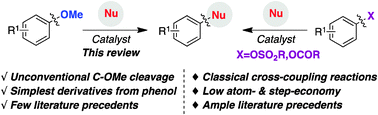
In 1979, the seminal work of Wenkert set the standards for the utilization of aryl and vinyl ethers as coupling partners via C–O bond-cleavage. Although the topic remained dormant for almost three decades, the last few years have witnessed a renaissance in this area of expertise, experiencing an exponential growth and becoming a significant discipline within the cross-coupling arena. The means to utilize readily accessible aryl or vinyl ethers as counterparts does not only represent a practical, powerful and straightforward alternative to organic halides, but also constitutes an excellent opportunity to improve our chemical knowledge about a relatively unexplored area of expertise. This review summarizes the most significant developments in the area of C–O bond-cleavage when employing aryl or vinyl ethers, providing a detailed overview of the current state of the art and including future aspects, when applicable.

 Please wait while we load your content...
Please wait while we load your content...