“Click” reactions: a versatile toolbox for the synthesis of peptide-conjugates
Abstract
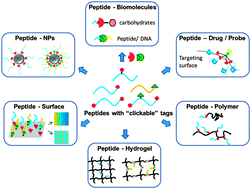
Peptides that comprise the functional subunits of proteins have been conjugated to versatile materials (biomolecules, polymers, surfaces and nanoparticles) in an effort to modulate cell responses, specific binding affinity and/or self-assembly behavior. However, the efficient and convenient synthesis of peptide-conjugates, especially the constructs with multiple types of peptide functionality remains challenging. In this critical review, we focus on “click” reactions that have been used to synthesis peptide-functionalized conjugates, introducing their reaction conditions, specifically elucidating parameters that influence reaction kinetics and total conversion, and highlighting examples that have been completed recently. Moreover, orthogonal “click” reactions that synthesize multi-functional biomaterials in a one-pot or sequential manner are noted. Through this review, a comprehensive understanding of “click” reactions aims to provide insight on how one might choose suitable “click” reactions to constitute peptide-functionalized molecules/surfaces/matrices for the development of advanced biomaterials.

 Please wait while we load your content...
Please wait while we load your content...