Amyloid-based nanosensors and nanodevices
Abstract
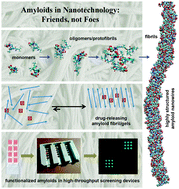
Self-assembling amyloid-like peptides and proteins give rise to promising biomaterials with potential applications in many fields. Amyloid structures are formed by the process of molecular recognition and self-assembly, wherein a peptide or protein monomer spontaneously self-associates into dimers and oligomers and subsequently into supramolecular aggregates, finally resulting in condensed fibrils. Mature amyloid fibrils possess a quasi-crystalline structure featuring a characteristic fiber diffraction pattern and have well-defined properties, in contrast to many amorphous protein aggregates that arise when proteins misfold. Core sequences of four to seven amino acids have been identified within natural amyloid proteins. They are capable to form amyloid fibers and fibrils and have been used as amyloid model structures, simplifying the investigations on amyloid structures due to their small size. Recent studies have highlighted the use of self-assembled amyloid-based fibers as nanomaterials. Here, we discuss the latest advances and the major challenges in developing amyloids for future applications in nanotechnology and nanomedicine, with the focus on development of sensors to study protein–ligand interactions.

 Please wait while we load your content...
Please wait while we load your content...