Guanidines: from classical approaches to efficient catalytic syntheses
Abstract
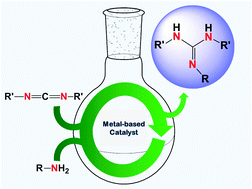
From organosuperbases capable of base-catalyzing organic reactions, through versatile ‘ligand-sets’ for use in coordination chemistry, to fundamental entities in medicinal chemistry, guanidines are amongst the most interesting, attractive, valuable, and versatile organic molecules. Since the discovery of these compounds, synthetic chemists have developed new methodologies that are mainly based on multi-step and stoichiometric reactions. Despite the fact that these methodologies are still being used by the interested scientific and industrial communities, drawbacks such as the poor availability of precursors, low yields, and use and production of undesirable substances highlight the need for safe, simple and efficient syntheses of these entities. This review focuses on the metal-mediated catalytic addition of amines to carbodiimides as an atom-economical alternative to the classical synthesis.

 Please wait while we load your content...
Please wait while we load your content...