Crystallization of molecular systems from solution: phase diagrams, supersaturation and other basic concepts†
Abstract
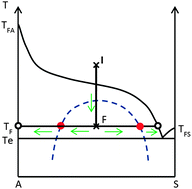
The aim of the tutorial review is to show that any crystallization from solution is guided by stable or metastable equilibria and thus can be rationalized by using phase diagrams. Crystallization conducted by cooling, by evaporation and by anti-solvent addition is mainly considered. The driving force of crystallization is quantified and the occurrence of transient metastable states is logically explained by looking at the pathways of crystallization and the progressive segregation which might occur in a heterogeneous system.
- This article is part of the themed collection: Nucleation and crystallisation

 Please wait while we load your content...
Please wait while we load your content...