Copper catalysed Ullmann type chemistry: from mechanistic aspects to modern development
Abstract
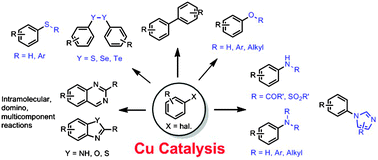
Cu-catalysed arylation reactions devoted to the formation of C–C and C–heteroatom bonds (Ullmann-type couplings) have acquired great importance in the last decade. This review discusses the history and development of coupling reactions between aryl halides and various classes of nucleophiles, focusing mostly on the different mechanisms proposed through the years. Selected mechanistic investigations are treated more in depth than others. For example, evidence in favour or against radical mechanisms is discussed. Cu(I) and Cu(III) complexes involved in the Ullmann reaction and N/O selectivity in aminoalcohol arylation are discussed. A separate section has been dedicated to the synthesis of heterocyclic rings through intramolecular couplings. Finally, recent developments in green chemistry for these reactions, such as reactions in aqueous media and heterogeneous catalysis, have also been reviewed.

 Please wait while we load your content...
Please wait while we load your content...