Photodynamics of Zr-based MOFs: effect of explosive nitroaromatics†
Abstract
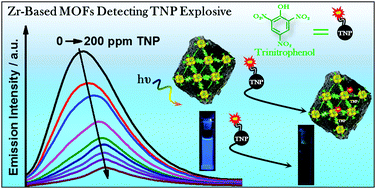
The present work describes the spectroscopic and photodynamics of two different Zr mixed-linkers MOFs (Zr-NDC/Tz and Zr-NDC/CN) and their interaction with nitroaromatics. Both MOFs exhibit comparable spectroscopic behaviours, with a broad emission band mainly due to the naphthalene excimers within their three-dimensional structure. Flash photolysis experiments show a slow radiative electron–hole (e−–h+) recombination, reflected as a large negative absorption band. The interaction with the selected nitroaromatic compounds produces a static fluorescence quenching of Zr-NDC/Tz. Interestingly, the addition of trinitrophenol (TNP) induces the formation of a charge-transfer complex, helped by intermolecular H-bonds formation, as shown by the steady-state and ps-time-resolved emission experiments. Remarkably, the (e−–h+) recombination is strongly affected due to the inhibition of the ligand-to-cluster charge transfer process within the MOF. The quenching constants for the nitroaromatics lacking –OH groups are in the order of 102 M−1, while it is two orders of magnitude higher for the TNP (1.8 × 104 M−1). Both MOFs are highly selective toward TNP. We also demonstrate the possibility to recycle these MOFs without significant loses in their ability to detect TNP. Our findings give the clues to understand the fluorescence quenching mechanism of new Zr-based MOFs in presence of explosive-like molecules, opening the way to improve these nanomaterials as highly selective sensor of nitroaromatics.


 Please wait while we load your content...
Please wait while we load your content...