Electrostatics determine vibrational frequency shifts in hydrogen bonded complexes†
Abstract
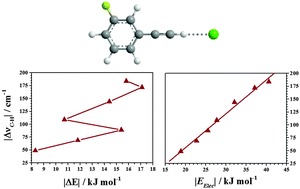
The red-shifts in the acetylenic C–H stretching vibration of C–H⋯X (X = O, N) hydrogen-bonded complexes increase with an increase in the basicity of the Lewis base. Analysis of various components of stabilization energy suggests that the observed red-shifts are correlated with the electrostatic component of the stabilization energy, while the dispersion modulates the stabilization energy.
- This article is part of the themed collection: PCCP Emerging Investigator Lectureship Award Winners

 Please wait while we load your content...
Please wait while we load your content...