Solvent effects on the intramolecular conversion of trimethylsulfonium chloride to dimethyl sulfide and methyl chloride†
Abstract
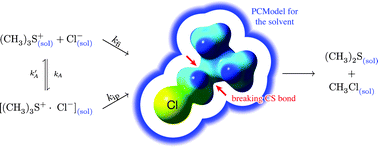
The formation of CH3Cl from (CH3)3SCl in various solvents has been studied based on M05/6-311+G(2d,p) DFT calculations to quantify the influence of the solvent on the stability of sulfonium cations. Four different pathways (one SN1, one backside and two frontside attacks for SN2) as well as the formation of different ion pairs (tripod, seesaw, and linear) are discussed to investigate the origin of the kinetic solvent effect (KSE) and the contribution of ion pairs to the overall reaction. Ion pairs are formed only in solvents with a permittivity ε lower than 28, but the reaction proceeds via a standard SN2 mechanism with a backside attack in all solvents. The formation of ion pairs does not change the order of the rate law, but it strongly influences the KSE, which can distinguish between reactions starting from free ions and those starting from ion pairs, in contrast to standard kinetic analysis.
- This article is part of the themed collection: Density functional theory and its applications

 Please wait while we load your content...
Please wait while we load your content...