Adsorption properties of nitrogen dioxide on hybrid carbon and boron-nitride nanotubes†
Abstract
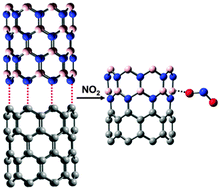
The properties of pristine carbon nanotubes (CNTs) can be modified in a number of different ways: covalent attachments, substitutional doping, induced defects, and non-covalent interactions with ligands. One unconventional approach is to combine CNTs with boron-nitride nanotubes (BNNTs) to form hybrid carbon and boron-nitride nanotube (CBNNT) materials. In this work, we perform a first-principles density functional theory study on the adsorption properties of NO2 on CBNNT heterostructures. It is found that the adsorption of NO2 is significantly increased on both zigzag CBNNT(8,0) and armchair CBNNT(6,6), as compared to either a pristine CNT or BNNT. For example, the chemisorption of NO2 on CNT(8,0) is found to be endothermic, while the chemisorption of NO2 on CBNNT(8,0) is an exothermic process with a very large binding energy of −27.74 kcal mol−1. Furthermore, the binding of NO2 on both CBNNT(8,0) and CBNNT(6,6) induces an increase in the conductivity of the nanotube. These characteristics indicate that the CBNNT heterostructures may have significant potential as an NO2 sensor or as a catalyst for NO2 decomposition reactions. Our calculations provide critical information for further evaluation, such as molecular-level adsorption simulations and microkinetic studies.

 Please wait while we load your content...
Please wait while we load your content...