Electron driven reactions in sulphur containing analogues of uracil: the case of 2-thiouracil
Abstract
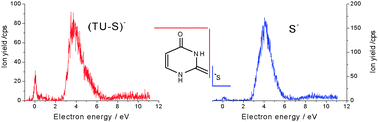
The fragmentation of 2-thiouracil (TU) molecules induced by low energy (<12 eV) electrons is investigated experimentally and theoretically. It is observed that most of the damage is localised at the sulphur site and in particular visible via the production of the thiocyanate, SCN−, anion. Similar to the canonical nucleobases the loss of the hydrogen atom is a predominant dissociation channel already at the subexcitation energies. The theory shows that for incident electron energies below 3 eV dissociative electron attachment is initiated by shape resonances implicating the π* molecular orbitals. It may also arise from the dipole bound supported state as illustrated by the production of the SCN−, S− and (TU–S)− fragments observed close to 0 eV but also the formation of (TU–H)− species at 0.7 and 1 eV.

 Please wait while we load your content...
Please wait while we load your content...