Insights into hydrophobic molecule release from polyelectrolyte multilayer films using in situ and ex situ techniques†
Abstract
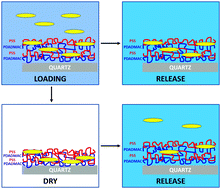
We report on the loading and release of curcumin (a hydrophobic polyphenol with anti-inflammatory and anti-bacterial properties) from polyelectrolyte multilayers composed of poly(diallyldimethylammonium chloride) (PDADMAC) and poly(sodium 4-styrenesulfonate) (PSS). We have used the in situ techniques of attenuated total reflectance (ATR) FTIR spectroscopy and quartz crystal microbalance with dissipation monitoring (QCM-D) to study the formation of the PEM and the incorporation of curcumin, providing direct evidence of the incorporation, in terms of molecular vibrations and gravimetric detection. The release of curcumin was followed using ex situ measurements of UV-visible spectroscopy of PEM films on quartz plates, in addition to in situ ATR FTIR measurements. Release was studied as a function of salt concentration of the release solution (0.001 M NaCl; 1 M NaCl). UV-visible spectroscopy indicated that salt concentration of the release solution had a major impact on release rates, with higher salt giving faster/more extensive release. However, prolonged timescale immersion and monitoring with UV-visible spectroscopy indicated that sample dehydration/rehydration cycling (required to measure UV absorbance) was responsible for the release of curcumin, rather than immersion time. In situ measurements of release kinetics with ATR FTIR confirmed that release does not occur spontaneously while the multilayer remains hydrated.

 Please wait while we load your content...
Please wait while we load your content...