Monophasic ligand-free alloy nanoparticle synthesis determinants during pulsed laser ablation of bulk alloy and consolidated microparticles in water†
Abstract
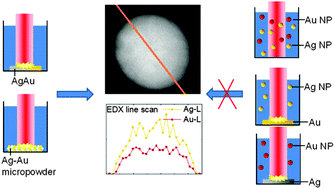
Chemical syntheses of homogenous solid solution alloy nanoparticles of noble metals require high temperature above 100 °C. Beside this, aqueous co-reduction methods lead to phase separation. In contrast, pulsed laser ablation in liquid (PLAL) allows synthesis of alloy nanoparticles with totally homogeneous ultrastructure in aqueous media at room temperature without reducing agents or organic ligands. However, to date, the dominant alloy formation process during PLAL is not fully understood. Based on the model of Ag–Au alloy, we elucidate that the underlying mechanism is not affected by post-irradiation or interactions with colloidal particles in solution but is caused directly by ablation. In this context we analyzed nanoparticles generated from alloy targets with 9 different compositions as well as pure Ag and Au references using UV-Vis spectroscopy, TEM and TEM-EDX line scans. The obtained results highlight that the total composition but not the microstructure of the applied target is the dominant parameter ruling elemental composition in the resulting solid solution alloy nanoparticles. Based on these findings, the application of pressed targets of metal powder mixtures in a continuous laser process with residence time <60 s allows economical fabrication of alloy nanoparticles ideally suited for applications in catalysis or biomedicine.

 Please wait while we load your content...
Please wait while we load your content...