Performance improvement and failure mechanism of LiNi0.5Mn1.5O4/graphite cells with biphenyl additive†
Abstract
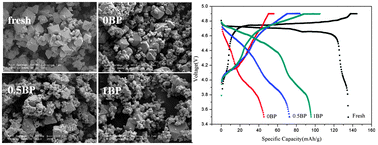
The cycling performance of the LiNi0.5Mn1.5O4/graphite cells cycled to 4.8 V versus Li/Li+ at room temperature has been investigated. The control electrolyte is 1.0 M LiPF6 ethylene carbonate (EC)/ethylmethyl carbonate (EMC)/fluoroethylene carbonate (FEC) (10/70/20, v/v) with 2 wt% lithium bis(oxalate)borate (LiBOB). Biphenyl (BP) (0.5 wt% and 1 wt%) is employed as the in situ electrochemical coating additive for LiNi0.5Mn1.5O4 cathode. The results indicate the potential window of electrolyte without BP is up to 5.5 V versus Li/Li+ on Pt electrode, but still decomposed seriously on both cathode and anode surfaces during prolonged cycles. The improved cycling performance with added 0.5 wt% BP in the control electrolyte can be attributed to the in situ electrochemical coating film on LiNi0.5Mn1.5O4 surface derived from BP electro-polymerization, which decreases the Mn dissolution and the lattice changes of LiNi0.5Mn1.5O4 cathode, and further inhibits Mn deposition and additional SEI formation on graphite surface. However, the thicker and compact BP electro-polymerization film using 1BP electrolyte on the cathode surface might inhibit the lithium intercalation/deintercalation and increase the polarization, then accelerates the capacity fading.

 Please wait while we load your content...
Please wait while we load your content...