First observation of HO˙ reactivity in water under high energy ions at elevated temperature
Abstract
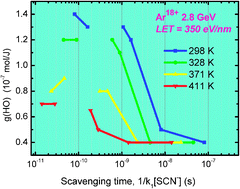
This communication reports the first observation of the formation of HO˙ produced under two different High energy ion beams, 18O8+ and 36Ar18+ having Linear Energy Transfers (LET) of 65 and 350 eV nm−1 respectively, at temperatures up to 411 K. Both scavenging with various concentrations of SCN− and heavy-ion pulse radiolysis methods are used with an original temperature and pressure regulated optical cell. Deconvolution of kinetics is used to analyze the evolution of HO˙ track segment yields as a function of time and temperature. It takes care of involving the ionic strength effect and Arrhenius expression in the rate constants correction. The results show a fast decay of HO˙ yields in the 10−10–10−8 s range which denotes an efficient reactivity of this species in the track structure of the ion beam. This effect is enhanced with the lowest LET of O8+. Increasing the temperature also accelerates the decays for both ions. These observations are discussed in terms of temperature activation of reactions and the track structure exhibiting the formation of HO˙ in a “low LET” penumbra around the ionization tracks. HO˙ track segment yields at 100 ns, of 0.4 × 10−7 and 0.6 × 10−7 mol J−1, respectively for 350 and 65 eV nm−1, are not affected by temperature.

 Please wait while we load your content...
Please wait while we load your content...