Structural aspects of heteropolyacid microemulsions†
Abstract
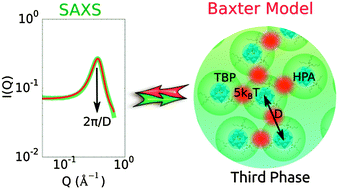
Metrical insights from X-ray scattering studies of dense fluid phases (known as “third” phases) in the Keggin heteropolyacid–tri-n-butyl phosphate (TBP)–n-alkane system are provided. Small-angle X-ray scattering (SAXS) experiments reveal inter-acid correlation peaks corresponding to average centre-of-mass to centre-of-mass separations of 18–23 Å between P⋯P, Si⋯Si, and Al⋯Al of H3PW12O40, H4SiW12O40, and H5AlW12O40, respectively, consistent with the presence of TBP solvates that form by hydrogen bonding between the acids and the phosphoryl group of TBP. The Baxter sticky sphere model analyses of the SAXS data reveal identical structures for all the dense phases with inter-cluster interaction energies of ∼5kBT. We demonstrate that the sticky sphere model is an essential paradigm for interpreting SAXS and predicting mesoscale assembly in heteropolyacid microemulsions. The model parameters for the ternary polyoxometalate–amphiphile–oil systems reveal, in rigorous clarity, how the interactions between heteropolyacid solvates underpin their condensation to produce the observed scattering data. Aside from aiding researchers in predicting the physical origins of SAXS in strongly-interacting micellar systems found in natural and engineered settings, such as chemical separations, our study provides mesostructural information that complements previously observed electrochemical behaviours for third phases formed by solvent extraction involving the contact of aqueous electrolytes of dodecatungsto-phosphoric, -silicic, and -aluminic acids with organic solutions (e.g. n-dodecane and n-octane) of TBP, and by simple dissolution of the acid salts of the polyoxometalate hydrates in the same organic solutions.

 Please wait while we load your content...
Please wait while we load your content...