Hydrogen bonding and proton transfer in cocrystals of 4,4′-bipyridyl and organic acids studied using nuclear quadrupole resonance
Abstract
Cocrystals of 4,4′-bipyridyl and several carboxylic acids were grown from the methanol solution of the cocrystal formers. Complete 14N NQR spectra of these cocrystals have been measured using 1H–14N nuclear quadrupole double resonance. The principal values of the quadrupole coupling tensor are calculated from the 14N NQR frequencies. A large variation in the 14N quadrupole coupling constant between 1.3 MHz and 4.7 MHz is observed. A very low 14N quadrupole coupling constant, characteristic for proton transfer O–H⋯N → O−⋯H–N+, is observed in 4,4′-bipyridyl–oxalic acid (1 : 1). In 4,4′-bipyridyl–5-chlorosalycilic acid (1 : 1) the 14N NQR data show the presence of a short, strong N⋯H⋯O hydrogen bond. A correlation of the principal values of the 14N quadrupole coupling tensor is observed. The correlation is analyzed in the model, where the deformation of the lone pair electron orbital and the change of the population of the π-electron orbital produce the variation of the 14N quadrupole coupling tensor in the hydrogen bonded 4,4′-bipyridyl. The temperature variation of the 14N quadrupole coupling tensor in 4,4′-bipyridyl–5-chlorosalycilic acid (1 : 1) is analyzed. Proton displacement within the N⋯H⋯O hydrogen bond and the change of the population of the π-electron orbital at the two nitrogen positions in a 4,4′-bipyridyl molecule in the temperature interval between 157 K and 323 K are determined.
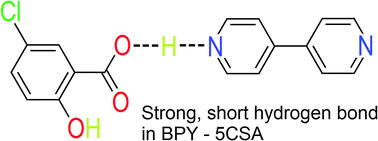

 Please wait while we load your content...
Please wait while we load your content...